Our group focuses on the development of new reactions that are either inspired by or directed toward natural products. Although technical discoveries are captivating, we are equally passionate about training next generation scientists. Skills and techniques obtained in our group are: Creative Thinking & Problem Solving, Independence & Leadership, Teamwork & Mentorship, Technology & Presentation, Synthesis Planning & Execution, Compound Isolation & Purification, Spectral Manipulation & Interpretation, Mechanism Discussion & Understanding.
Often referred to as the central science, chemistry continues to demonstrate wide-ranging utility. New reaction development maintains a privileged status due to extensive applications derived from organic synthesis. Since pioneering syntheses of urea (1828), acetic acid (1845), and glucose (1891), organic synthesis has impacted countless technological advances.

For centuries and perhaps even millennia, human beings have mined the natural world for remedies and cures. Now common medicinal agents such as aspirin, quinine, penicillin, taxol, and vancomycin have been used to fight pain, malaria, bacterial infections, cancer, and antibiotic resistant strains of bacteria, respectively. Until recently, humanity was dependent upon the isolation of natural products directly from the environment. Beginning in the early 1800’s, chemists began to unravel the mystery of organic synthesis and, in the last century, thousands of reactions have been invented that allow for synthetic construction of useful natural and unnatural products. The total synthesis of natural products is a vibrant area of research which requires critical thinking, tenacity, and a bit of good fortune. However, it was not until R. B. Woodward and E. J. Corey, both Nobel Laureates, arrived on the scene that this field truly launched. The audacity of Woodward to attack complex natural products long before modern instrumentation was available followed by the brilliance of Corey to formalize “retrosynthetic analysis” laid a foundation for future synthetic organic chemists.

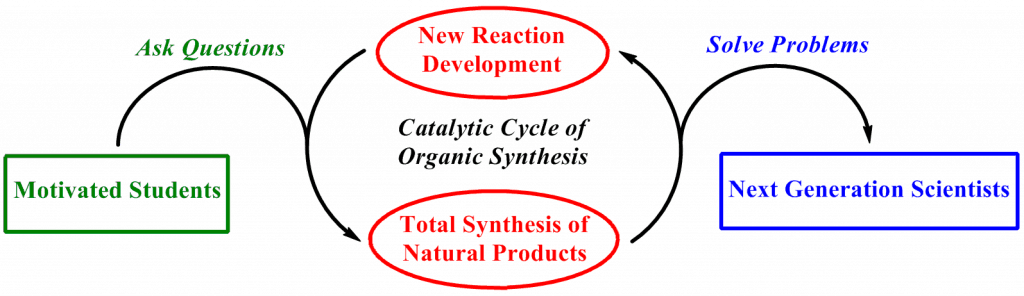
As students enter a “catalytic cycle” of organic synthesis, they are trained to solve problems and, more importantly, ask important questions. We seek to develop new reactions that can be applied toward the synthesis of natural products. Conversely, as we pursue the synthesis of a natural product, we serendipitously discover new reactions that likely would have remained undiscovered. These novel findings may be applicable to the project at hand or something unanticipated. Louis Pasteur noted that “in the fields of observation, chance favors only the prepared mind,” so we train students to observe and understand why a reaction failed to afford the desired product. Often the greatest discoveries were made by astute observations rather than perfect hypotheses. Ultimately, motivated students will be transformed by this “catalytic cycle” into next generation scientists.
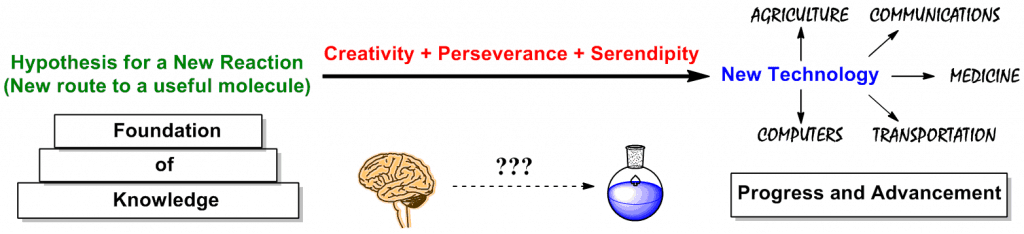
On one side of the organic synthesis coin is the development of new synthetic methods in which we devise a hypothesis for a new reaction based upon a foundation of knowledge. We then test that hypothesis (sometimes in many different ways) and creativity, perseverance, and serendipity pays off with the development of new technology. These new reactions may be useful in areas such as agriculture, communications, medicine, transportation, or computers, thus contributing to human progress.
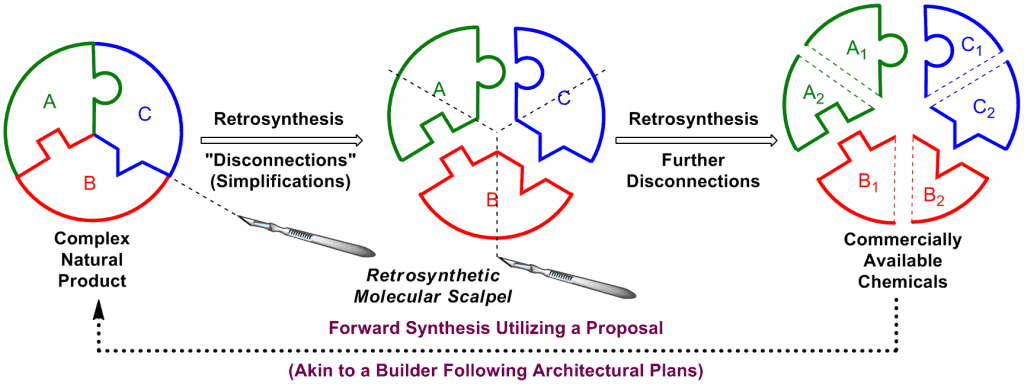
On the flip-side of the coin, upon initial evaluation of a complex natural product, the endeavor of total synthesis seems daunting. However, retrosynthetic analysis is the key to unlocking the mystery of each peculiar natural product. Solely upon inspection of the chemical structure as represented on paper, we sever this complex target molecule through “disconnections” into smaller and more manageable sub-target fragments A, B, and C, which can be simplified further with our “molecular scalpel” ultimately leading to commercially available chemicals. These commercially available chemicals are then arranged in a forward synthetic sense utilizing a proposal, akin to a builder following architectural plans. It is only then that we enter the arena of the laboratory and begin to carry out the synthetic plan. Undoubtedly, this retrosynthetic analysis will be revised many times when inevitable roadblocks are encountered in the laboratory synthesis.