Research in the lab is focused on understanding cellular immune responses, and uncovering the tricks that pathogens and parasites use to overcome them. We use the fruit fly Drosophila melanogaster and parasitoid wasps that infect flies as a model host-parasite system.

Parasitoid wasp approaching a larva 
Parasitoid infecting a larva (Photo credit: Todd Schlenke)
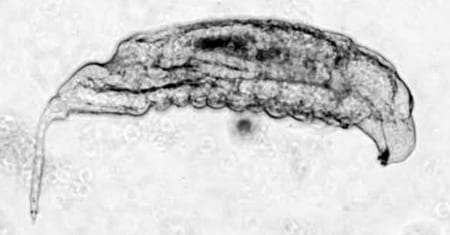
In this system, parasitoid wasps infect Drosophila melanogaster larvae, triggering a cellular immune response in the fly. In this response, Drosophila immune cells surround the wasp egg and become melanized in a process known as melanotic encapsulation. This response kills the invading wasp and allows the fly to continue development.
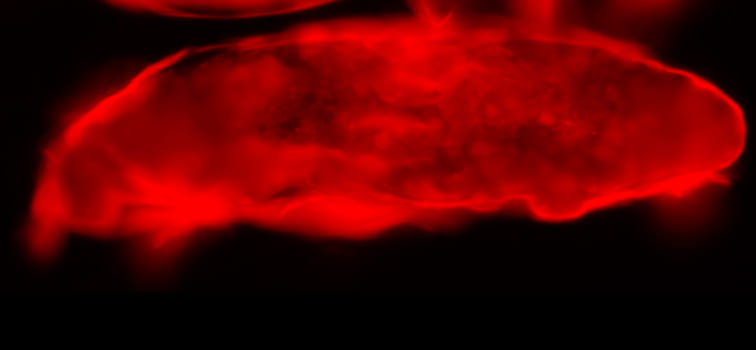
Immune cells (red) surrounding an egg 
Larva with encapsulated wasp eggs
However, wasps attempt to block the immune response by injecting venom into the fly during infection. Wasp venoms contain proteins that manipulate host signaling and can inhibit the immune response, allowing the wasp to evade the encapsulation response and successfully parasitize the fly.
Our work is described in more detail below:
Regulation of host signaling by parasitoid venom proteins
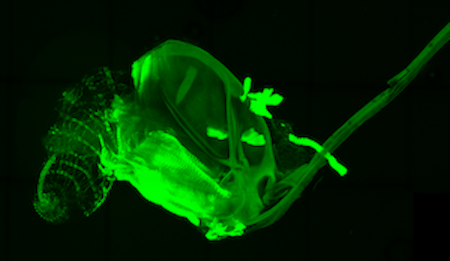
In order to successfully parasitize the fly host, the parasitoid wasp must overcome the fly’s cellular immune response. Parasitoids accomplish this using virulence factors found in their venoms. Venom proteins are produced in the venom gland and manipulate cell signaling in the host leading to immune evasion, altered metabolism and developmental delays.
The tightly controlled regulation of signaling events is important to maintain cell function, and the pathogenesis of multiple diseases arises due to the deregulation of signaling pathways. The goal of our research is to gain new insight into the regulation of signaling pathways by characterizing the signal manipulation activities of invading parasitoids in the Drosophila-parasitoid wasp model host-parasite system. This research will shed light on how signal regulation mechanisms function and the roles that multiple pathways play in health and disease.
Venom gland stained with αSERCA 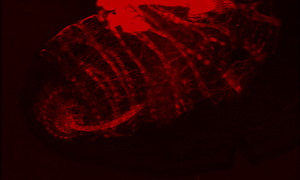
Venom gland stained with phalloidin
The role of innate immunity in Alzheimer’s Disease pathogenesis
Alzheimer’s Disease is a common disease that causes dementia in patients and affects 5.7 million Americans. Our proposed research will investigate the recently discovered links between Alzheimer’s Disease and immunity using a simple model to understand the role of Alzheimer’s Disease genes in the immune response to pathogen infection. By understanding this interaction, we hope to shed light on how immunity and infection can affect the development of Alzheimer’s Disease and identify potential targets for the advancement of new therapies.
Molecular genetics of Drosophila melanogaster cellular immunity
In the encapsulation response, fly immune cells activate highly conserved signaling pathways including the JAK-STAT and JNK pathways. These pathways regulate common cell biological processes including cell differentiation, activation, migration and adhesion. We are interested in understanding the molecular genetic basis of the various stages of the encapsulation response, and multiple projects in the lab are geared towards this goal.
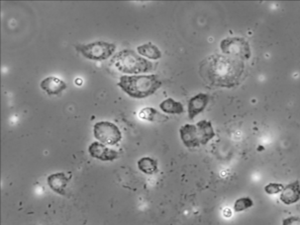
Immune cells 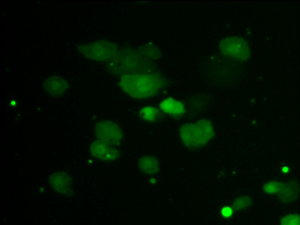
JAK-STAT pathway activity 
Immune cells 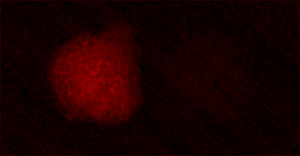
JNK pathway activity
Innate immune receptors: missing-self recognition, self-tolerance and autoimmunity
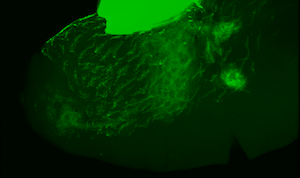
We are particularly interested in understanding how innate immune cells discriminate between self tissue and pathogens. In mounting an effective immune response, a host’s immune cells must both target the pathogen and prevent autoimmune damage. To gain insight into this process, we are investigating the genetic architecture of several Drosophila autoimmune mutants. These mutant flies mount a melanotic encapsulation response against their own tissues in the absence of wasp infection. By understanding the basis for missing-self recognition in these mutants, we will begin to uncover the underlying genetic basis of self tolerance and autoimmunity.
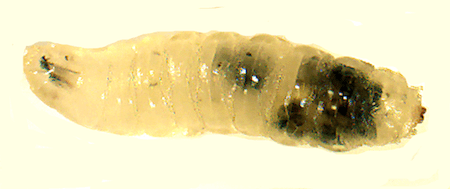
Larva showing encapsulation of self tissue 
Immune cells (red) targeting self tissue