I showed that in the field that Aedes aegypti is competitively inferior to Aedes albopictus, particularly when food resources are scarce, even though A. aegypti are virtually unparasitized. These results indicate that resource competition is sufficient to account for replacement of A. aegypti, and that shared parasites need not be involved. Gregarine parasites do seem to affect mosquito ability, and Brianna Aliabadi (M.S. student) and I showed that A. albopictus at recently colonized sites have low parasite loads and improved competitive ability.
For more details on this research topic, please see:
Lounibos, LP, SA Juliano. 2018.Where vectors collide: The importance of mechanisms shaping the realized niche for modeling ranges of invasive Aedes mosquitoes. Biological Invasions 20:1913–1929 (Elton Review – Invited).
Camara, DCP, CT Codeço, SA Juliano, LP Lounibos, TIS Riback, GR Pereira, NA Honorio. 2016. Seasonal differences in density but similar competitive impact of Aedes albopictus (Skuse) on Aedes aegypti (L.) in Rio de Janeiro, Brazil. PLOS One 11(6): e0157120 http://dx.doi.org/10.1371/journal.pone.0157120
Juliano, SA, LP Lounibos. 2016. Invasions by mosquitoes: the roles of behaviour across the life cycle. in, JS Weis & D Sol, eds. Biological Invasions and Animal Behaviour. Cambridge Univ. Press, pp. 245-265
Leisnham, PT, SA Juliano. 2010. Interpopulation differences in competitive effect and response of the mosquito Aedes aegypti and resistance to invasion of a superior competitor. Oecologia 164:221–230 http://link.springer.com/article/10.1007/s00442-010-1624-2/fulltext.html
Leisnham, PT, LP Lounibos, GF O’Meara, & SA Juliano. 2009. Interpopulation divergence in competitive interactions of the mosquito Aedes albopictus. Ecology 90:2405–2413.
Costanzo, K.S., Kesavaraju, B., & Juliano, S.A. 2005. Condition-specific competition in container mosquitoes: the role of non-competing life-history stages. Ecology 86:3289-3295.
Braks M.A.H., Honório N.A., Lounibos L.P., Lourenço-de-Oliveira R, & Juliano SA. 2004. Interspecific competition between two invasive species of container mosquitoes, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazil. Annals of the Entomological Society of America 97:130-139.
Braks M.A.H., Honório, N.A., Lourenço-de-Oliveira, R., Juliano, S.A. & Lounibos, L.P. 2003. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in southeastern Brazil and Florida, USA. Journal of Medical Entomology 40:785-794
Aliabadi, B. K. & S. A. Juliano. 2002. Escape from Gregarine Parasites Affects the Competitive Impact of an Invasive Mosquito Biological Invasions 4:283-297
Juliano, S. A. 1998. Species introduction and replacement among mosquitoes: Interspecific resource competition or apparent competition? Ecology 79:255-268.
I have also been investigating variation in the outcome of this invasion. In most of Florida, Aedes albopictus has displaced Aedes aegypti, particularly in cemeteries, where both species colonize water-filled vases. However, in urban areas of South Florida (Tampa, Ft. Myers, Miami), A. aegypti persists, despite invasion by Aedes albopictus. At nearby cemeteries in suburban or rural areas (LaBelle, Arcadia, Lakeland, Bartow), A. aegypt has gone extinct. One hypothesis for this difference in outcome is that the impact of competition from A. albopictus on A. aegypti is different at these urban sites, perhaps because of differences in the aquatic environment. To test this, I have run competition experiments at 3 cemeteries where the two coexist, and at 3 cemeteries where A. aegypti has gone extinct following invasion by A. albopictus. In both 1999 and 2000 the results of these experiments were similar and clear. Competition from A. albopictus has the same effect on A. aegypti at both coexistence and extinction sites. Thus, the difference in outcome of invasion is not a result of differences in the aquatic environment and may be related to differences in the aerial envrionment of adults and eggs (e.g., desiccation, host availability). Future work funded by the NIH grant will investigate these possibilities through a combination of experiments, modeling, and observation.
Juliano, S.A., Lounibos, L.P., O’Meara, G.F. 2004. A field test for competitive effects of Aedes albopictus on Aedes aegypti in South Florida: Differences between sites of coexistence and exclusion? Oecologia 139:583-593.
Juliano, S. A., G. F. O’Meara, J. R. Morrill, & M. M. Cutwa 2002. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia 130:458-469.
Effects of Habitat Drying on Populations and Communities
We have also investigated effects of food availability on population increase in Aedes triseriatus and the effect of habitat drying and previous resource depletion by earlier cohorts of A. triseriatus on the quality of food resources. Andrea Aspbury and I showed that drying of the habitat had significant detrimental effects on resource quality for Aedes triseriatus. Further, we demonstrated that intraspecific competitive effects of A. triseriatus could be expressed even among cohorts of mosquitoes that did not overlap in time. These effects seem to be mediated by depletion of resources within containers.
For more details on this research topic, please see these publications:
Westby, KM, SA Juliano. 2017. No detectable role for predators mediating effects of aquatic habitat size and permanence on populations and communities of container-dwelling mosquitoes. Ecological Entomology 42:439-448 http://onlinelibrary.wiley.com/doi/10.1111/een.12405/epdf
O’Neal, PA, SA Juliano. 2013. Seasonal variation in competition and coexistence of Aedes mosquitoes: stabilizing effects of egg mortality or equalizing effects of resources? Journal of Animal Ecology 82:256–265 http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2656.2012.02017.x/full
Aspbury, A. S. & Juliano, S. A. 1998. Negative effects of drying and prior exploitation on the detritus resource in an ephemeral aquatic habitat. Oecologia 115:137-148. © Springer-Verlag
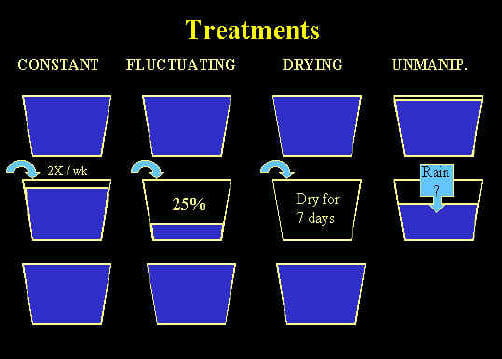

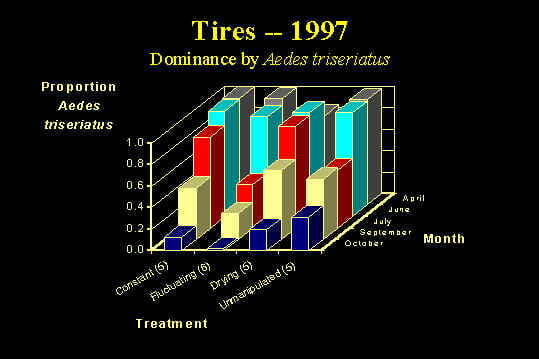
Along with my previous work on habitat drying and metamorphosis, these studies of drying led to a new research direction – a project examining the effects of habitat drying on the entire community of container invertebrates – which is a current focus of my research. This project is involved a multi-year field manipulation of the drying regime of natural tree holes and artificial containers (discarded tires). We monitored abundances of 8 species of mosquitoes, about 15 species of other invertebrates (including copepods, mites, and cladocera) in our experimental containers and ultimately will determine the role of habitat drying and fluctuation in determining community compostition. Our results (after 1 field season of manipulation) show that pattern of water input to tires affects demography of A. triseriatus, the dominance of A. triseriatus within the community, and production of pupae of A. triseriatus and Anopheles barberi. After two years of manipulation, we found that drying regime affected community composition in tires late in the year, with containers that dry out lacking predatory taxa (copepods, A. barberi, and Toxorhynchites rutilus) and having lower species number overall.